SOTERIA® BED BARRIER
Soteria Bed Barrier: Re–engineering Clean for Patient Surfaces
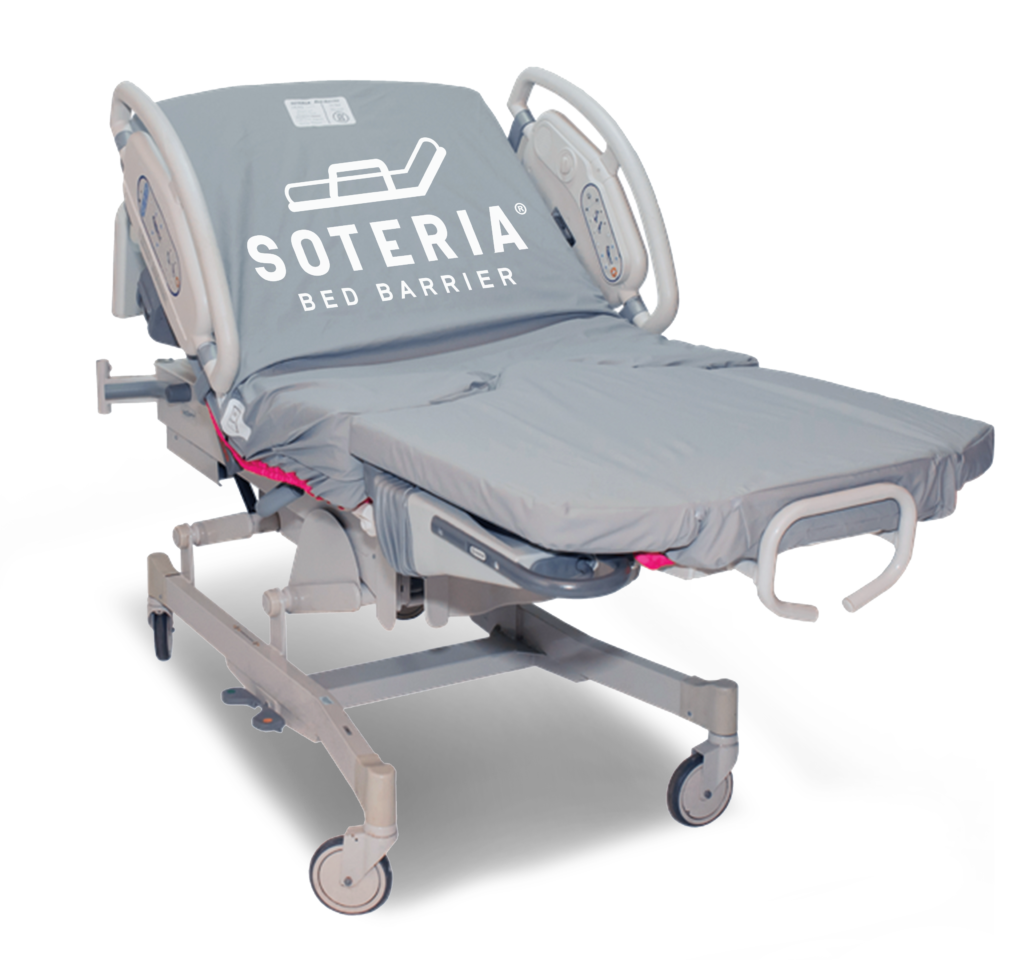
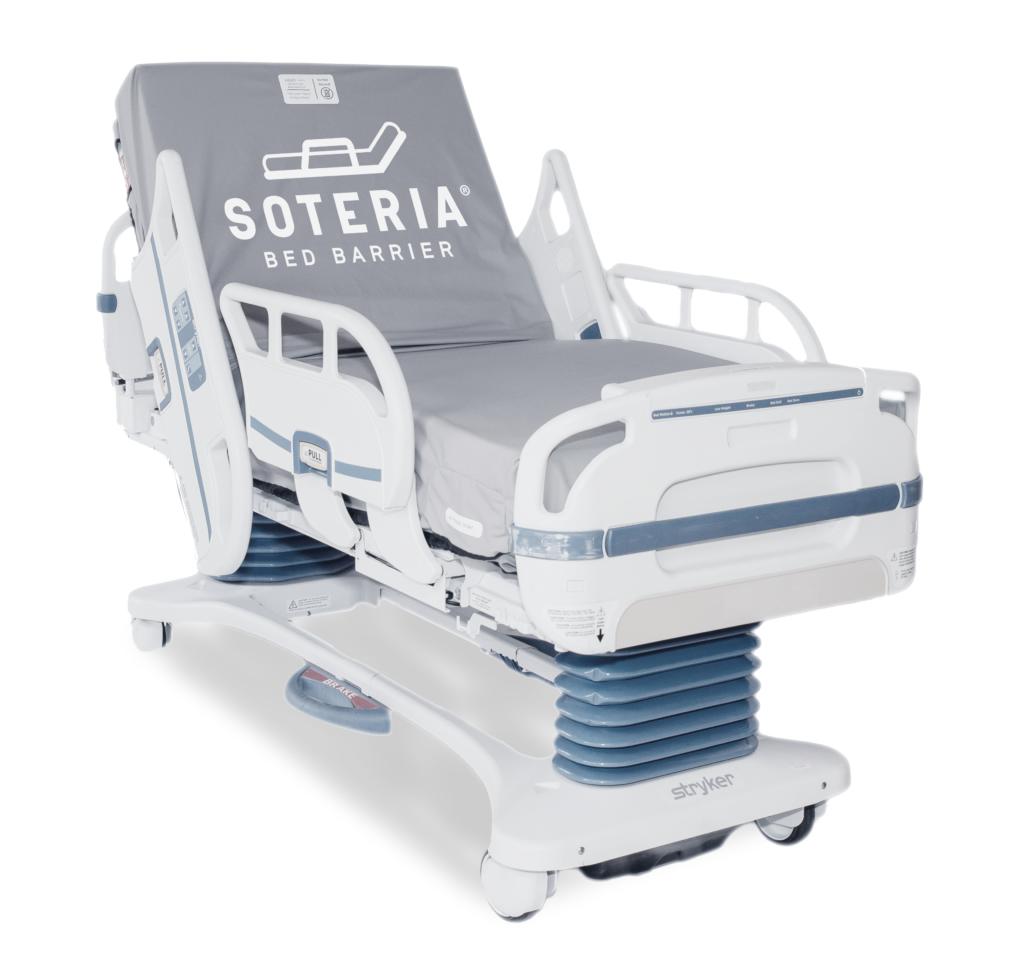
The Soteria® Bed Barrier is the first of its kind launderable and reusable healthcare bed barrier that provides a protective physical barrier to protect the bed and mattress from patient soiling, helping to reduce contamination during use. Designed for acute and long-term care beds and stretchers, it fits securely over the mattress and bed deck and attaches to the bed frame without disrupting bed and mattress performance during patient care.
What Makes Soteria Different?
The Soteria Bed Barrier is classified as a Level 3 barrier per AAMI Standard PB70; this is similar to other protective equipment such as protective apparel and drapes intended for use in healthcare facilities. The Soteria proprietary surface formulation allows for laundering that meets AMMI TIR 12 acceptance criteria for high level disinfection plus spore removal (99.9999%) using a rigorous commercial healthcare laundry method.
FDA 510-K Cleared
More than a mattress cover, the Soteria Bed Barrier defines a new category for surface protection between mattress and patient. Its innovative and patented features, including a proprietary laundry process that allows for re-use after laundering, was cleared with the assignment of a unique FDA product code (QTV).

MEETS CDC GUIDELINES
Soteria meets CDC Guidelines for Environmental Infection Control in Health-Care Facilities, specifically Section G providing a clean surface for every patient. The Soteria Bed Barrier is validated for 150 laundry cycles. Both the embedded RFID-chip and bar-code enable tracking of laundry cycles and are used in our propriety Ecosystem, an automated workflow, barrier, inventory tracking system.acking of laundry cycles and inventory management.
CDC guidelines say use of a barrier protective covering can be used as appropriate for noncritical equipment surfaces that are touched frequently by gloved hands during the delivery of patient care likely to be contaminated with blood or body substances and difficult to clean.

TIME SAVINGS
Unlike manual processes that require 3-6 steps per the manufacturer’s instructions for use, Soteria is easy to apply and remove between patients. It eliminates multiple steps and provides a cleaner surface. Additionally, Soteria is light table inspected at the laundry, after each laundering, providing assurance of a sound patient surface while eliminating the need for mattress inspections between patients by hospital staff.

COST SAVINGS
Soteria pays for itself through reduced labor and material costs, and it eliminates the need to replace outer mattress covers every 1-2 years when they reach their expected life. By protecting the mattress surface with a sound barrier, it protects the mattress core from patient fluids saving on premature mattress replacements and disposal costs due to fluid ingress. Soteria’s unique design also protects the bed deck from soiling and reduces contaminates helping to reduce the need for harsh chemicals to clean and disinfect this area that can cause rust.
Soteria® Bed Barriers are available for most major brands, including the newest therapeutic beds and mattresses.

CLEANING VALIDATION
Soteria is designed to withstand the mechanical action of commercial washer/extractors as well as high heat and chlorine. The robust laundry process performed at the hospital laundry removes residual protein and hemoglobin below the FDA Reprocessing Guidance as well as 99.9999% of test organisms including C. diff. After the laundry wash cycle, Soteria is rinsed with a neutralizing additive before final extraction bringing the barrier to a pH neutral condition and then dried at 160 degrees. After each laundering, the barrier is inspected on a light table then individually wrapped before returning to the facility. Soteria’s laundry process removes stains (including blood and betadine) and leaves the barrier smelling fresh and clean.