Soteria Bed Barrier
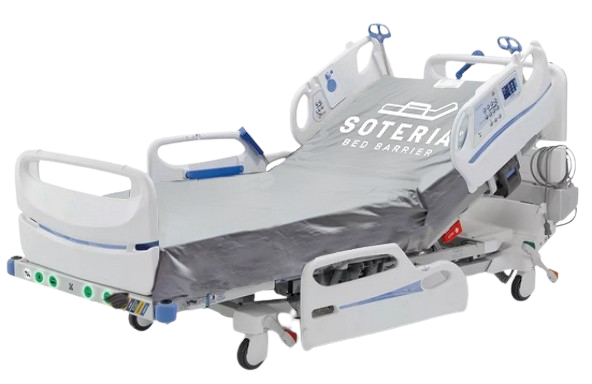 The Soteria Bed Barrier, may look like a fitted sheet but it is the only FDA-cleared microbiological barrier that provides a protective physical barrier to protect the hospital bed and mattress from patient soiling. Clinical studies indicate Soteria helps to reduce contamination that can transmit Healthcare-Acquired Infections (HAIs) including CMS-designated Never Events.
The Soteria Bed Barrier, may look like a fitted sheet but it is the only FDA-cleared microbiological barrier that provides a protective physical barrier to protect the hospital bed and mattress from patient soiling. Clinical studies indicate Soteria helps to reduce contamination that can transmit Healthcare-Acquired Infections (HAIs) including CMS-designated Never Events.
The Soteria Bed Barrier is launderable and reusable, and available for beds used throughout healthcare facilities.
It provides a level of clean equivalent to FDA 2015 reprocessing requirements for high level disinfection (log 6 or 99.9999% kill of both vegetative bacteria and mycobacteria, as well as spore reduction).
Mattress and bed deck reprocessing time is significantly reduced using Soteria because its disinfecting-equivalency step happens in the laundry, not during room turn-over.
SOTERIA OFFERINGS FOR HOSPITAL MATTRESSES
Soteria Bed Barriers are available for all healthcare beds produced by major manufacturers such as Hill-Rom, Stryker, and others.
Additionally, Soteria Neonatal Microbiological Mattress Barriers are available for neonatal warmers and incubators produced by all major manufacturers. In fact, the Soteria Neonatal Microbiological Mattress Barrier is included in a major manufacture's Instructions for Use for its' warmers and incubators.
Soteria® Panda Warmer mattress barrier is patent protected by no. D1065875. Soteria® Giraffe Incubator mattress is patent protected by no D1065876.
Whether you are looking for a Manufacturer’s-IFU-Compliant approach to reduce room turn time by Environmental Services or are seeking assistance to improve Patient Safety, talk to Trinity Guardion about how the Soteria Bed Barrier can help.
PROBLEM / SOLUTION FOR
HOSPITAL MATTRESSES AND BED DECKS
There are dozens of published clinical studies that indicate that hospital beds and mattresses, even after terminal cleaning at patient discharge, are not really Clean. Likewise, there are dozens of studies that prove that “unclean” beds are associated with transmission of HAIs.
Despite repeated emphasis on the importance of mattress and bed reprocessing and disinfection by FDA, CDC, CMS, the AMA, ECRI, and the Joint Commission, it remains a problem. Just in the spring of 2024, the Veteran’s Administration issued a Patient Safety Advisory:
“Undetected fluid ingress and egress from inpatient and outpatient mattresses can lead to hospital-associated infections, patient tissue degradation, and/or patient death.”
Healthcare facilities throughout the country are challenged every day with keeping patients safe from the #1 patient touchpoint in a facility – the hospital mattress.
THE BENEFITS OF A SOTERIA BED BARRIER
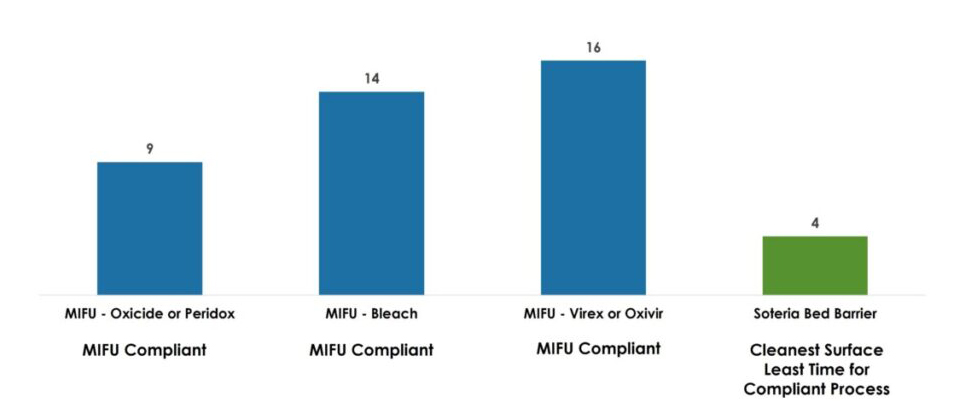
Soteria simplifies hospital mattress reprocessing, saving time in room turn-time while providing a log 6 or high-level disinfection equivalency for enhanced patient safety.
3 PROOFS FOR SOTERIA BED BARRIERS
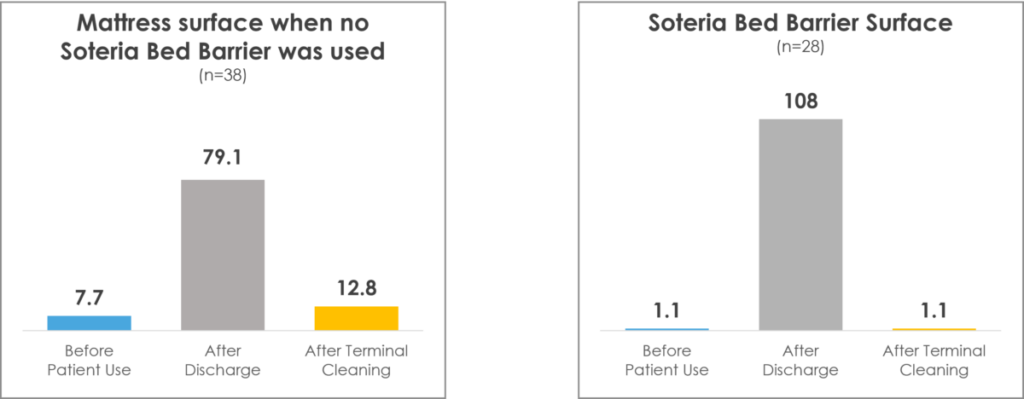
In a randomized clinical study, Soteria was proven to reduce mattress pathogens significantly at terminal cleaning as opposed to sheets alone.
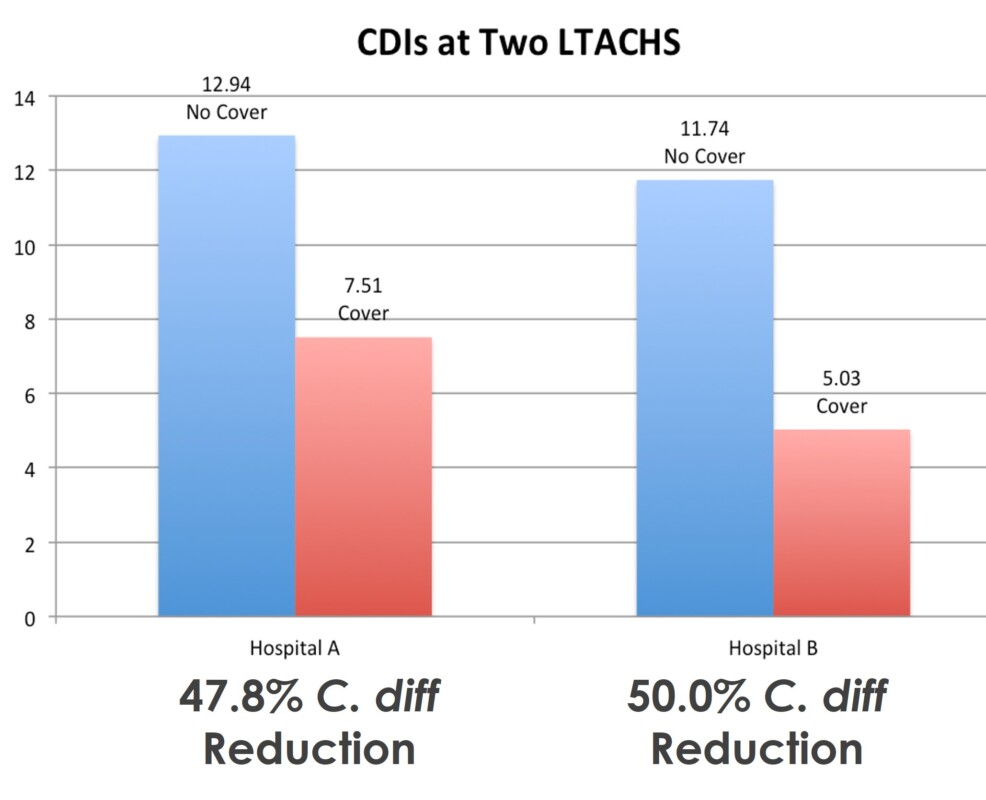
A peer-reviewed clinical study demonstrated use of Soteria reduced C.diff by approximately 50% into LTAC hospital units; these units were considered to have low levels of C.diff prior to the study.
HOW WE ENHANCE PATIENT SAFETY: THE IMPACT OF THE SOTERIA® LAUNDRY PROCESS
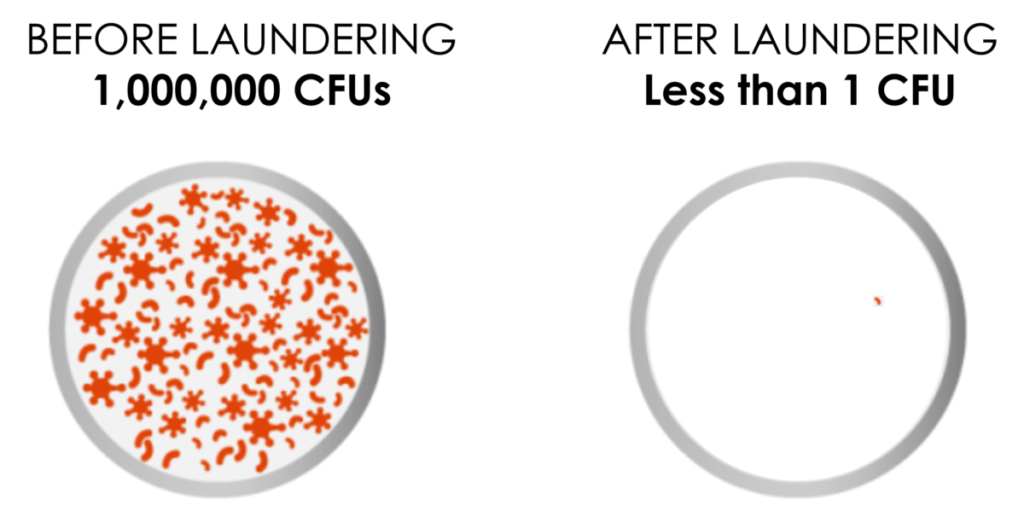
Soteria is designed to make it easier for healthcare facilities to help keep patients safe by minimizing contagious pathogens and spores remaining after terminal cleaning. It does this via a validated laundry process which provides a high-level disinfectant-equivalent level of clean (Log 6 or 99.9999%).
Contact us for more information on how Soteria can help both with your patient safety initiatives focused on HAI reduction and with reducing your mattress reprocessing time with a simple, replicable process and proven product.