
Can I use a 1-step process to clean/disinfect my hospital mattresses?
No. Research shows that 1-step processes achieve less than a 90% reduction in harmful bacteria on mattresses.38 Using the broadest interpretation, the FDA recommends that mattress reprocessing achieves at least a 99.9999% reduction in vegetative bacteria.23,24 A 1-step process can also leave residual chemicals on the mattress surface, a concern for patient safety.39 Additionally, 1-step processes can cause premature damage to your mattresses, which may void your warranty and expose patients to additional risk.17,18 Not only this, but a 1-step process of simply wiping the mattress with a disinfectant falls well short of the processes recommended by manufacturers, which usually involve 5-6 steps. This includes pre-cleaning visible soil, cleaning non-visible soil, rinsing off the cleaning agent, disinfecting with an approved disinfectant, rinsing off the disinfectant, allowing the mattress to dry, and visually inspecting the mattress for damage.40-42 Current Joint Commission regulations require hospitals to follow the manufacturer’s instructions for use. This includes following the MIFU for the disinfectant used in the process.

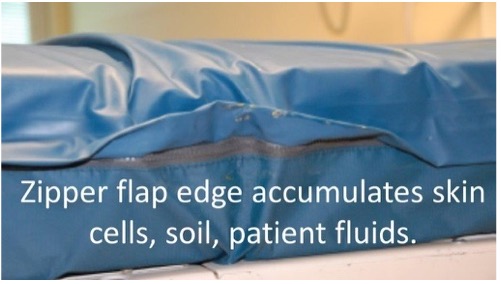
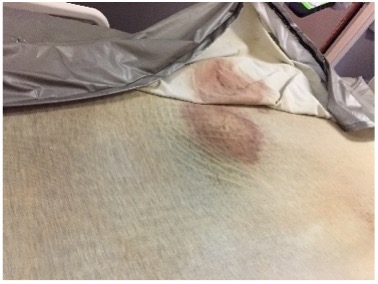
Picture 1 and picture 2 Bioburden can build up along sewn seams and under zipper flaps of mattress covers if not precleaned before disinfecting. Picture 3 Disinfectants not rinsed off the mattress cover can cause damage and allow body fluids to leak through to the mattress core.
FOLLOW-UP: Perform a mattress audit at your facility to determine if any of your mattresses
have been compromised. The FDA recommends removing any damaged, worn, or visibly stained mattresses according to facility procedures and MIFUs and replacing mattress covers that have visible signs of stains, damage, or wear.16 The bed deck should be checked for rust as well.
References
23. Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling. fda.gov. https://www.fda.gov/media/80265/download. Published March 17, 2015. Updated June 9, 2017. Accessed September 11, 2020.
24. Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency. fda.gov. https://www.fda.gov/media/136533/download. Published March 2020. Accessed September 11, 2020.
38. Hooker EA, Allen S, Gray L, et al. A randomized trial to evaluate a launderable bed protection system for hospital beds. Antimicrob Resist Infect Control. 2012;1(27). doi:10.1186/2047-2994-1-27
39. Best Practices for Cleansing, Disinfecting, and Care of Polyurethane Support Surface Covers. National Pressure Injury Advisory Panel. https://cdn.ymaws.com/npiap.com/resource/resmgr/s3i/S3i_NPIAP_Cleansing_Disinfec.pdf. Published 2020. Accessed April 13, 2021.
40. Cleaning and Disinfecting Instruction Video (REV 1). Hillrom. https://www.hill-rom.com/international/Education/education-by/e-learning/cleaning-instructions/. Accessed September 9, 2020.
41. Stryker® Maintenance Manual: Isolibrium™ Support Surface (REV B). Stryker. https://techweb.stryker.com/Support_Surfaces/2971/0815/maintenance/2971-009-002B.pdf. Published 2017. Accessed September 4, 2020.
42. User Manual and Technical Description: Eleganza 5 Positionable Bed for Intensive Care and Opticare Integrated Mattress Replacement System (Version 6). Linet. Published October 2018.
