Hospital Mattress Infection Prevention
Mattresses Documented as a Patient Safety Concern
Hospital Mattresses and Beds Are Not Adequately Disinfected Between Patients
Mattresses and beds are the most common touchpoints for patients and move throughout the hospital. The average acute care bed supports 80 patients annually and travels through at least three rooms or units monthly.6-8 Hospital mattresses and beds are also a common caregiver touchpoint. In 2020, the Center for Disease Control (CDC) added surfaces to its hand hygiene emphasis, recognizing the importance of cleaning and disinfecting environmental surfaces to prevent healthcare-acquired infections.9
As Class I or Class II medical devices, the FDA requires that all hospital beds and mattresses must be cleaned and disinfected at patient discharge (terminal cleaning) before the bed can be used for the next patient.10 Device manufacturers must provide a validated process that is technically feasible to clean and disinfect medical devices to a level of disinfection consistent with the device’s intended use.11
A common misperception is that beds are non-critical devices, thus needing only low-level disinfection. FDA and CDC guidance suggests that devices that come into contact with blood or bodily fluids receive intermediate disinfection. Furthermore, devices that come into contact with spores and non-intact skin (for example, incontinence-associated dermatitis) require high-level disinfection. Surfaces such as mattresses for neonates with undeveloped skin also require high-level disinfection.4,10
As a result, prevalent mattress cleaning/reprocessing at terminal discharge is insufficient and may undermine other infection-prevention strategies throughout an institution. Many studies have shown that mattresses remain contaminated even after terminal cleaning.12
A prospective multicenter trial of 23 acute care hospitals by Carling, Parry, and von Beheren identified the thoroughness of terminal cleaning as an average of 49% of surfaces.13 In a MRSA study, Blythe et al. identified environmental contamination occurring in the rooms of 73% of infected patients and 69% of colonized patients.14
Hooker et al. collected samples from 38 hospital beds using traditional-sheets-only to determine the level of mattress contamination after terminal cleaning. Their results showed an insufficient reduction in FDA-required disinfection of vegetative bacteria. Furthermore, they identified several pathogens in their samples that are known to be critical contributors to HAIs, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE).12 This cleaning failure can lead to increased transfer of multi- drug-resistant organisms (MDRO) and infections to subsequent patients.
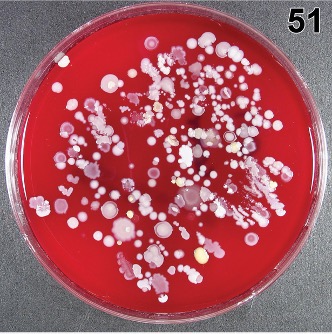
Bacteria Cultured from “Clean” Mattress after Terminal Cleaning.11
Insufficient disinfection at terminal cleaning is a critical patient safety issue. By not achieving the threshold for low-level disinfection, the remaining bacteria can recolonize to 42% of the pre-treatment level in just 6.5 hours.15
Contaminated Mattresses and HAIs
Contaminated Mattresses have been Proven to Increase HAIs
There are numerous studies linking patient infections to the previous mattress occupant. Cohen et al. found that a patient is 5.83 times more likely to get an HAI if the previous bed occupant had an infection.2 Witt et al. found that exposure to a bed previously used by a patient with Clostridium difficile (C. diff) confers a 50% greater risk of contracting a C. diff infection for the next patient using that bed, even though they used quaternary disinfectants and bleach for terminal cleaning. They also found that viable spores were still present three months after the initial C. diff patient.16
Bousquet et al. linked 4 mattresses to an Enterobacter cloacae outbreak in their intensive care unit (ICU) that lasted four months, killed 4 patients and infected 14 others.16 Van der Mee-Marquet et al. reported that contaminated mattresses were the source of an outbreak of Multi-drug-resistant Enterobacter cloacae in an ICU, with 15 patients colonized or infected.18
Reboux evaluated the impact of an alternative disinfection process of steam pulverization on bacterial colonization on incubator mattresses after concluding chemical disinfection methods were insufficient. Even after using steam pulverization, 90% of mattresses were still contaminated, compared to 100% before. During this study, three neonates developed late onset sepsis (LOS).19
Several studies have confirmed the same disturbing fact: Previous bed occupants in the same room with an HAI increase a patient’s risk of acquiring an infection.2,16
Challenges in Mattress Reprocessing
Why are Hospital Mattresses so Challenging to Clean
Over the past 50 years, mattresses have evolved significantly. To help prevent pressure injuries, manufacturers began producing softer, air-permeable mattresses with polyurethane coatings rather than the original hard, non-porous surface. When the mattress is new, the fabric coating is fluid-proof while still allowing moisture vapor to pass through; however, that soft, porous fabric is much thinner and more easily damaged.1
Often, the chemicals used to clean and disinfect are incompatible with the mattresses’ soft surfaces, compromising the integrity of the fabric coating and leading to premature damage.4,7 In fact, none of the EPA’s Registered Antimicrobial Products effectiveagainst Clostridioides difficile (C. diff) Spores [List K] are recommended for use on soft, porous surfaces.19 However, concerns over C. diff infections have led many hospitals to continue to utilize bleach on mattresses, which damages the mattress and shortens its usable life. Damaged mattresses allow bodily fluids to seep in and out of the mattress core, making it impossible to decontaminate.10,21
Microbes have also evolved with the biofilms they produce. These biofilms encapsulate pathogens in a protective matrix, making it even more difficult to clean beds.22
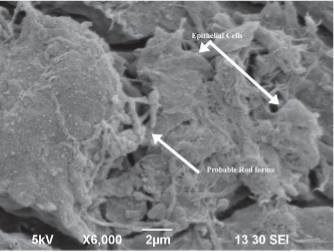
SEM-image: Bacteria in fissures on mattress surface caused by disinfectant damage.
Bacteria Travels through Fissures from Surface to Mattress Core
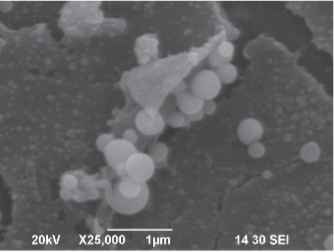
SEM-Image: Staph on patient-side of integrated mattress cover.
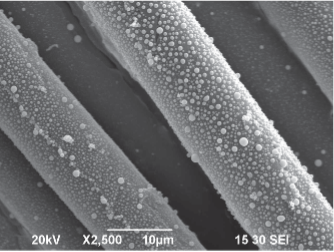
SEM-image: Staph on bottom of integrated mattress cover.
Moreover, another major contributor to the lack of adequate disinfection is that most hospitals report using a one-step “wipe-and-walk” process.3 The one-step wipe-and-walk is a violation of mattress reprocessing MIFUs and does not meet FDA guidance for any level of disinfection.4,15,23,24
For perspective, bed and mattress manufacturers must validate a vegetative bacteria disinfection process for their products. Accomplishing this level of disinfection typically takes 5-6 steps. There are separate steps for cleaning and disinfection, with dwell time a significant aspect of efficacy. Rinsing with water is required between steps.23,24
The choice of one-step cleaning is usually based on misunderstanding disinfection process requirements and trying to accelerate room turn time.
Studies have shown, however, that this one-step reprocessing achieves less than a 90% reduction in harmful bacteria and no reduction in spores, leaving the mattress still contaminated. It can also leave residual chemicals on the mattress surface, further compounding patient safety concerns.12
This lack of sufficient mattress cleaning and disinfection has prompted calls for action.
Witt concluded the 2023 study commenting that new technologies to better eradicate C. diff spores from a bed may lead to significant reductions in hospital-onset CDIs (HO-CDIs).16 Similarly, Reboux concluded:
“Even if previous studies have reported the advantages of steam pulverization in comparison with chemical methods for incubator disinfection, the present study did not show a better antimicrobial efficacy. However, the present results highlight the urgent need to develop an effective disinfection method, especially for mattresses that represent a primary reservoir for pathogen involved in LOS. Thus, there is a need for substantial efforts and consultation of manufacturers to rethink incubator design and mattress conception, and to reevaluate the composition of materials used.”19
Solutions for Mattress Reprocessing to Keep Patients Safe
Solutions to Keep Patients Safe
Regulations today allow hospitals only two options for reprocessing beds and mattresses during terminal cleaning. They can either (1) follow the multi-step MIFU for mattress cleaning and reprocessing provided by the bed and mattress manufacturer or (2) use a new microbiological barrier and follow its MIFU.2,8 In fact, the FDA created a unique product code for a microbiological barrier, and to date, only one product qualifies: the Soteria® Bed Barrier by Trinity Guardian.2
Bed Barrier Data
So what is a Bed Barrier, and How Does it Enhance Patient Safety
A launderable bed barrier (FDA product code QTV) provides a physical barrier (level 4 barrier per AAMI Standard PB70) to separate the patient from the mattress. Additionally, a bed barrier protects the mattress and bed deck from patient soiling, helping to reduce contamination during use. The fabric is breathable, reducing skin maceration concerns.
The unique microbiological bed barrier currently available for healthcare mattresses has a proprietary and validated laundry process that can be accomplished in-house or by outsourced laundries. It uses the same washer and similar process used for launderable surgical linens, including light-table inspection for damage.25
Most importantly, it is an engineered solution that removes through laundering >99.9999% of vegetative bacteria, mycobacteria, and spores. This process was tested and found effective in addressing numerous types of bacteria and spores associated with HAIs, including including pseudomonas, MRSA, C. diff, and Klebsiella.12
Bed barrier. Label is enlarged to show detail.
Mattress Contamination Risk is Significantly Reduced Using a Repeatable, Documented Laundry Process Instead of Manual Wipe-Down
Several real-world studies have validated the effectiveness of an FDA-approved bed barrier in preventing pathogen transmission. One randomized prospective study showed that CFUs (Colony-Forming Units) on mattresses increased from 7.7 on admission to 12.8 after terminal cleaning. When a microbiological bed barrier was used, there was no significant change in the bacterial counts on the surface of the mattress from admission to after terminal cleaning.12
Bed barrier usage reduced bacteria and spore levels to their pre-use level, significantly below those of the mattress and sheets cohort.12
The decreased CFUs and contamination of mattresses when using microbiological bed barriers have also been proven to correlate directly with reduced HAIs. A study in two long-term acute care hospitals (LTCAH) assessed the rate of C. diff infections. Both hospitals showed a significant reduction of approximately 50% when controlling for patient length of stay and hand washing. While this study specifically focused on C. diff, researchers support the logical conclusion that using a launderable mattress cover will likely decrease other HAIs secondary to environmental transmissions, such as MRSA and VRE infections.26
Conclusion
Mattress contamination is often an overlooked yet critical patient safety issue. For several reasons, current disinfecting processes are insufficient and frequently undermine other infection prevention strategies. A launderable mattress barrier provides an evidence-based solution for healthcare facilities looking to keep patients safe while remaining compliant with infection prevention guidelines.11,25
| At-Risk Populations to Consider for Additional Mattress Safety Measures: | NICUs and nurseries Wound or burn care units Oncology Patients with known or suspected C. diff infections MRSA isolation rooms |
References
- Hooker EA. Hospital mattress failures—A hidden patient danger. Infect Control Hosp Epidemiol. 2023;44(3):501-503. doi:10.1017/ice.2021.486.
- Cohen B, Liu J, Cohen AR, Larson E. Association Between Healthcare-Associated Infection and Exposure to Hospital Roommates and Previous Bed Occupants with the Same Organism. Infect Control Hosp Epidemiol. May 2018;39(5):541-546. doi:10.1017/ice.2018.22.
- Hooker EA. Disinfecting hospital beds and mattresses: A time for change. American Journal of Infection Control. 2021;49(10):1341. doi:10.1016/j. ajic.2021.07.020.
- Guidelines for Environmental Infection Control in Healthcare Facilities. Centers for Disease Control and Prevention. Updated July 2019. Accessed November 15, 2024, https://www.cdc.gov/infection-control/media/pdfs/Guideline-Environmental-H.pdf.
- Soteria Bed Barrier (Trinity Guardion) (2022).
- Hospital Average Length of Stay by State. Definitive Healthcare. Accessed November 15 2024, https://www.definitivehc.com/resources/glossary/length-of-stay#:~:text=Length%20of%20stay%20(LOS)%20is,(heart%20attacks)%20and%20diabetes.
- Marks B, Haas Ed, Abboud T, Lam I, Datta I. Uncovering the rates of damaged patient bed and stretcher mattresses in Canadian acute care hospitals. Canadian Journal of Infection Control. 2018;33(3):171-175.
- Furness CD, Srigley JA, Gardam M. How much do beds and mattresses sleep around? Automated measurement of bed frame and mattress movement in an acute care hospital. Canadian Journal of Infection Control. 2017;32(4):222-224.
- Considerations for Reducing Risk: Surfaces in Healthcare Facilities Centers for Disease Control and Prevention. Accessed November 15, 2024, https://www.cdc.gov/healthcare-associated-infections/hcp/infection-control/index.html.
- FDA Covers for Hospital Bed Mattresses: Learn How to Keep Them Safe. U.S. Food & Drug Administration. December 1, 2024. Updated 11/20/2017. https://www.fda.gov/medical-devices/hospital-beds/covers-hospital-bed-mattresses-learn-how-keep-them-safe.
- Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling Guidance for Industry and Food and Drug Administration Staff. U.S Food & Drug Administration. Updated June 9, 2017. https://www.fda.gov/media/80265/download.
- Hooker EA, Allen S, Gray L, Kaufman C. A randomized trial to evaluate a launderable bed protection system for hospital beds. Antimicrobial Resistance and Infection Control. 2012/07/26 2012;1(1):27. doi:10.1186/2047-2994-1-27.
- Carling PC, Parry MF, Von Beheren SM. Identifying opportunities to enhance environmental cleaning in 23 acute care hospitals. Infect Control Hosp Epidemiol. Jan 2008;29(1):1-7. doi:10.1086/524329.
- Blythe D, Keenlyside D, Dawson SJ, Galloway A. Environmental contamination due to methicillin-resistant Staphylococcus aureus (MRSA). J Hosp Infect. Jan 1998;38(1):67-9. doi:10.1016/s0195-6701(98)90176-1.
- Recommendations for Disinfection and Sterilization in Healthcare Facilities. Centers for Disease Control and Prevention. Updated June 2024. Accessed November 15, 2024, https://www.cdc.gov/infection-control/hcp/disinfection-and-sterilization/index.html.
- Witt LS, Howard-Anderson J, Prakash-Asrani R, Overton E, Jacob JT. The role of the hospital bed in hospital-onset Clostridioides difficile: A retrospective study with mediation analysis. Infect Control Hosp Epidemiol. May 2024;45(5):599-603. doi:10.1017/ice.2023.254.
- Bousquet A, van der Mee-Marquet N, Dubost C, et al. Outbreak of CTX-M-15-producing Enterobacter cloacae associated with therapeutic beds and syphons in an intensive care unit. Am J Infect Control. Oct 1 2017;45(10):1160-1164. doi:10.1016/j.ajic.2017.04.010.
- van der Mee-Marquet N, Girard S, Lagarrigue F, et al. Multiresistant Enterobacter cloacae outbreak in an intensive care unit associated with therapeutic beds. Crit Care. Feb 2006;10(1):405. doi:10.1186/cc4835.
- Reboux M, Chavignon M, Tristan A, Plaisant F, Laurent F, Butin M. Disinfection of incubators in neonatal intensive care units: impact of steam pulverization on bacterial colonization. Antimicrobial Resistance & Infection Control. 2023/03/16 2023;12(1):18. doi:10.1186/s13756-023-01226-y.
- EPA’s Registered Antimicrobial Products Effective Against Clostridioides difficile (C. diff) Spores [List K]. United States Environmental Protection Agency. Updated June 3, 2024. Accessed December 1, 2024. https://www.epa.gov/pesticide-registration/epas-registered-antimicrobial- products-effective-against-clostridioides.
- Gilboa M, Houri-Levi E, Cohen C, et al. Environmental shedding of toxigenic Clostridioides difficile by asymptomatic carriers: A prospective observational study. Clin Microbiol Infect. Aug 2020;26(8):1052-1057. doi:10.1016/j.cmi.2019.12.011.
- Prinzi A, Rohde R. The Role of Bacterial Biofilms in Antimicrobial Resistance. The American Society for Microbiolog. Accessed November 18, 2024. https://asm.org/articles/2023/march/the-role-of-bacterial-biofilms-in-antimicrobial-re.
- P280 Mattress Instructions for Use. Hill-Rom Services, Inc. Updated October 2020. Accessed December 1, 2024. https://www.hillrom.co.uk/content/dam/hillrom-aem/emea/en/marketing/products/p280-mattress/documents/187692(10)_EN.pdf.
- IsoFlex LAL® Support Surface Operations/Maintenande Manual. Updated August 2017. Accessed December 1, 2024. https://techweb.stryker. com/Support_Surfaces/2860/IsoFlex/2860-109-001C.2.pdf
- Hooker EA, Ulrich D, Brooks D. Successful Removal of Clostridioides Difficile Spores and Pathogenic Bacteria From a Launderable Barrier Using a Commercial Laundry Process. Infectious Diseases: Research and Treatment. 2020;13:1178633720923657. doi:10.1177/1178633720923657.
- Hooker EA, Bochan M, Reiff TT, Blackwell C, Webb KW, Hart KW. Decreasing Clostridium difficile Healthcare-Associated Infections through Use of a Launderable Mattress Cover. American Journal of Infection Control. 2015;43(12):1326-1330. doi:10.1016/j.ajic.2015.07.002.
