SOTERIA® INFANT MATTRESS BARRIER REPROCESSING SERVICE
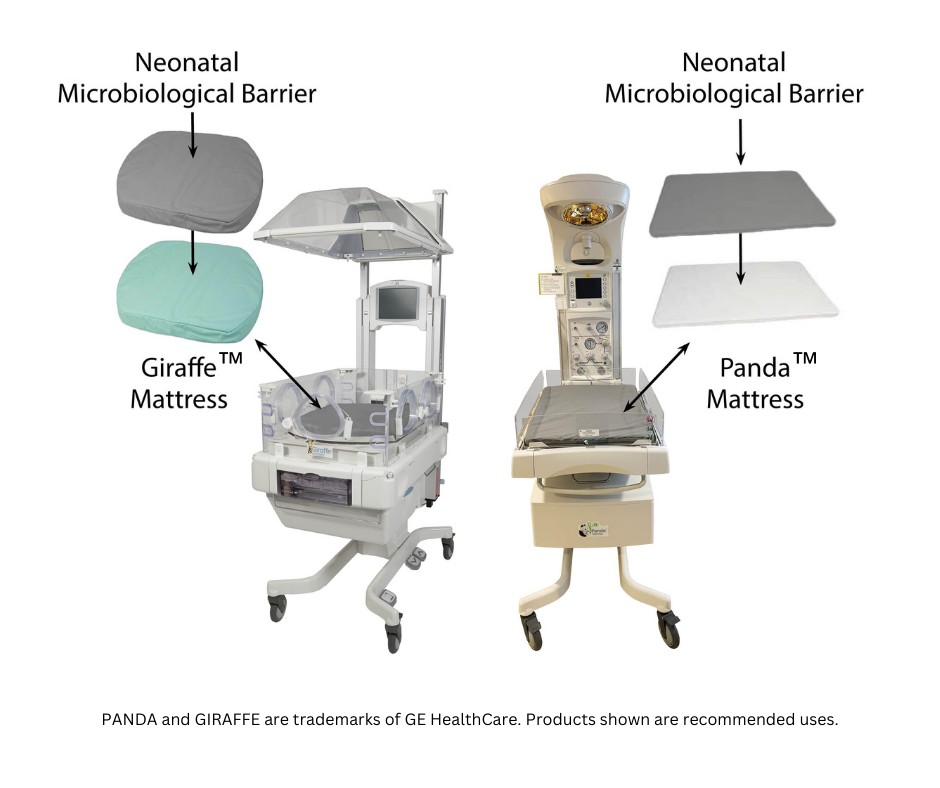
The Soteria® Infant Mattress Barrier is more than a hospital mattress cover. As easy to use as a fitted it sheet, it is the first and only protective barrier for infant care to help hospitals protect the equipment used for vulnerable newborns and babies:
Delivers the appropriate decontamination level of protection against mattress contamination.
Prevents microbe transfer through the surface.
Meets both PB70 and ASTM 1671 standards.
Is biocompatible per ISO 10993-1.
Will not introduce X-ray artifacts.
The Soteria Infant Bed Barrier also provides a protective physical barrier to protect the mattress in warmers and NICU incubators from patient soiling. Launderable, reusable and sustainable, it meets the endpoints defined by AMII TIR12 for high-level disinfection, washing away > 99.9999% bacteria including MRSA and spores, and has been integrated as a MIFU reprocessing option in a major manufacturer’s IFU (Instructions for Use).
Combined with our cloud-based inventory platform, Soteria helps hospitals manage usage, replacement, and compliance with ease.
This barrier cleaning service is integrated as part of this infant protection solution – to maximize ease of compliance, staff convenience, and infant protection.
Soteria takes the challenging steps in mattress reprocessing out of your facility... and puts it into ours. With the Soteria Infant Mattress Barrier, you get not just a unique product but a service for laundering as well.

What Makes Soteria Unique?

Proven Process
Preserves facility resources.
Following manufacturer’s instructions for use for bed and mattress reprocessing is required – and it can take a toll on your staff and your mattresses. Soteria significantly simplifies cleaning of hospital warmer and incubator mattresses. For our Infant Mattress Barriers, we take the challenging steps in mattress reprocessing out of your facility and into ours. It’s a process of Collect/Effect/Inspect/Direct/Protect.
You collect soiled Soteria Infant Mattress Barriers in a laundry bin provided specifically for that purpose and ship them to us. We effectively launder, inspect for material integrity with a light table in a clean room, individually pack and then ship the validated barrier directly back to your facility for you to use to protect patients and mattresses.
This innovative technology, combined with our advanced cloud-based Ecosystem, forms a powerful, dashboard-driven supply chain management and compliance solution designed for real-time tracking, resource allocation, and usage monitoring of our mattress barriers. Using Radio-frequency identification (RFID), the Ecosystem seamlessly tracks and locates barrier supplies in real-time ensuring that patients will always have a clean mattress surface to lie on

The One & Only
The only Infant Mattress Barrier recognized in MIFUs.
Uniquely, the Soteria Infant Mattress Barrier is actually presented as an option in the MIFUs of the leading manufacturer of infant warmers and incubators. While it is as easy to put on as a fitted sheet, it is actually classified as a barrier.

Unique Protection
Advanced materials and a proprietary laundry process provide protection.
Soteria uses advanced materials and a proprietary laundry process to bolster efficiencies in mattress reprocessing. This extends the realizable lifespan of mattresses which can break down if MIFU guidelines are not followed and the appropriate cleaning solutions/disinfectants are not used. It is more than a hospital mattress cover; it actually qualifies as a Level 4 barrier, like surgical gowns and drapes, meeting both AAMI Standard PB 70 and ASTM 1671 standards. Additionally, it is biocompatible per ISO 10993-1 and will not introduce X-ray artifacts.
It removes > 99.9999% of pathogens and spores, exceeding FDA Reprocessing Requirements.

For more information on our solution: Contact Bruce Rippe at brippe@trinityguardion.com or call 812-932-2601.
For more product information, go to Soteria Bed Barriers.
Soteria® Infant Mattress Barriers are available for most major brands and ALL GE HealthCare warmers and incubators...including Panda® Warmers & NICU incubators.
Giraffe® Incubator Carestation®

Giraffe® OmniBed® Carestation®
Panda® iRes Warmer
Panda, Giraffe, OmniBed, and Carestation are registered trademarks of GE HealthCare.
Trinity Guardion is not affiliated with or endorsed by GE HealthCare in any way.
Soteria® Panda Warmer mattress barrier is patent protected by no. D1065875. Soteria® Giraffe Incubator mattress is patent protected by no D1065876.

CLEANING VALIDATION
Soteria is designed to withstand the mechanical action of commercial washer/extractors as well as high heat and chlorine. The robust laundry process removes > 99.9999% of test organisms including C. diff. as well as removing residual protein and hemoglobin, exceeding FDA Reprocessing Requirements. After the laundry wash cycle, Soteria is rinsed with a neutralizing additive before final extraction bringing the barrier to a pH neutral condition and then dried at 160 degrees. After each laundering, the barrier is inspected on a light table, then individually wrapped before returning to the facility. Soteria’s laundry process removes stains (including blood and betadine) and leaves the barrier smelling fresh and clean.
