WOUND CARE
PROBLEM
- FDA Reusable Medical Device Regulations require device manufacturers to validate a six-log bacteria removal (99.9999%) on hospital beds and mattresses.
- Current disinfectants typically don’t achieve a one-log (90%) disinfected mattress surface. Bed linens and pads don’t prevent cross-contamination.
- Hard-surface disinfectants are often incompatible with mattress surfaces – challenges the disinfection process and causes mattress failures.
- Mattresses may not be inspected after every patient. Many hospitals have not fully audited for mattress failure. Your patients may be at risk today.
- Residual chemicals from one step cleaning processes can cause skin injuries.
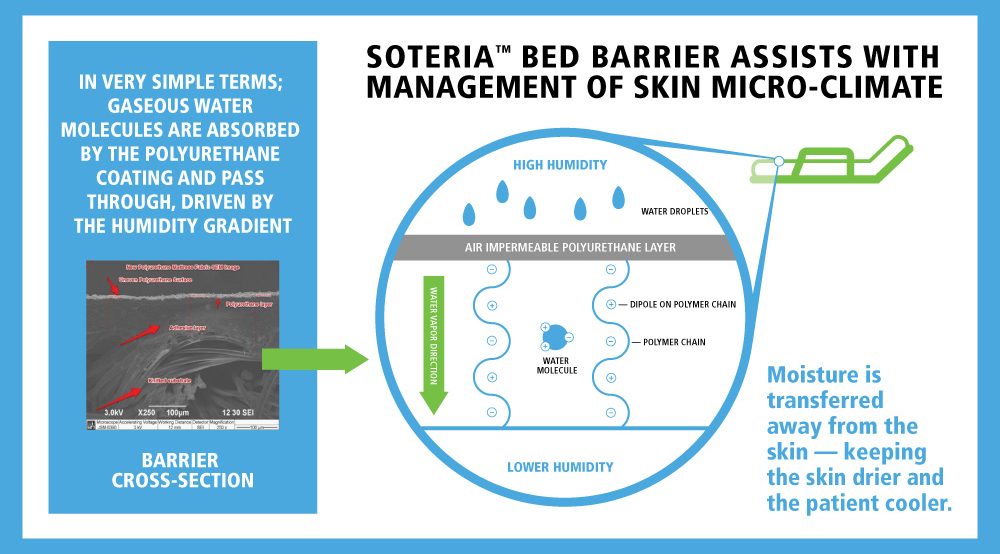
SOLUTION
- Mattress contamination risk is greatly reduced by the use of a repeatable, documented laundry process instead of manual wipe-down.
- Soteria® Bed Barrier is laundered after each patient – protects bed and mattress from patient fluids.
- The Soteria® Bed Barrier is laundered using high heat and a chlorine-based disinfection process that follows CDC guidelines for pathogenic organisms.
- After each laundering the barcoded bed barrier is inspected to ensure a sound surface for the next patient.
- Soteria® Bed Barrier is made of a breathable textile that blocks fluids, soils and microbes without interfering with wound care.

