Hospital Bed Reprocessing Failures
Introduction
Mattresses and beds impact all aspects of care, are the most common touchpoints for patients and move throughout a hospital. The average acute care bed supports 80 patients annually and travels through at least three rooms or units monthly.1-3 In 2020, the Centers for Disease Control (CDC) recognized environmental surfaces, in addition to hand hygiene, as a critical area of focus for infection prevention efforts.4
Health care full-body support surfaces—mattresses—and their reprocessing are creating an untenable situation for health care leadership committed to patient safety and efficient resource stewardship. Since the turn of the millennium, pressure injuries have significantly decreased due to various care initiatives, including the introduction of softer, porous mattresses. However, these mattresses present greater challenges in terms of cleaning and disinfection, especially when dealing with aggressive multidrug-resistant organisms (MDROs).
Current strategies for mattress reprocessing are falling short. Despite ongoing efforts, surface covers wear out faster than expected, compromising the mattress core. When the cover is damaged, fluid can leak in and out of the mattress, becoming a source of infection transmission and a biohazard for patients. This creates another significant patient safety issue and additional expense for the health care facility.
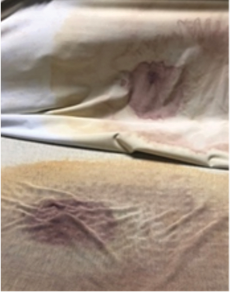
The images below highlight the critical issue: three mattresses with compromised covers that failed inspection. In each case, the attached mattress covers failed, allowing bodily fluids to penetrate the mattress core. Naturally, fluid movement can go both ways; fluids leaking into the mattress core can also seep out onto the next patient assigned to that bed.


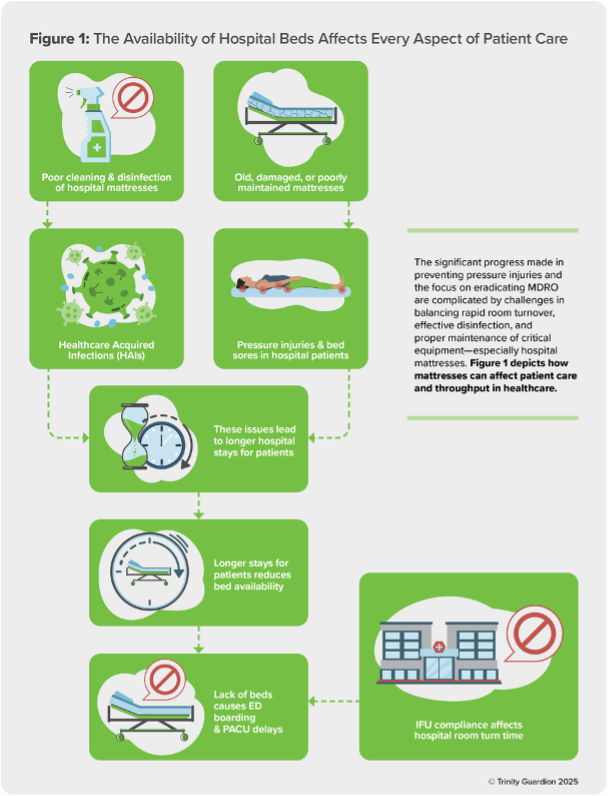
The significant progress made in preventing pressure injuries and the focus on eradicating MDROs are complicated by challenges in balancing rapid room turnover, effective disinfection and proper maintenance of critical equipment—especially hospital mattresses. Figure 1 illustrates how mattresses can impact patient care and throughput in health care.
Addressing Pressure Injuries with Softer Mattresses
In 2008, Centers for Medicare Services (CMS) designated pressure injuries in health care settings as “never events”—serious, preventable medical errors that should never occur.5 Preventing these incidents is crucial for patient safety and directly impacts reimbursement.6
To help prevent pressure injuries, bed and mattress manufacturers began producing softer, air- permeable mattresses with polyurethane coatings rather than the original hard, non-porous vinyl surface. The polyurethane surface can help reduce skin maceration by allowing moisture vapor to pass through, providing a cooler patient surface. When the mattress is new, the fabric coating is fluid-proof while still allowing moisture vapor to pass through; however, that soft, porous fabric is much more delicate, as the new covers are only 1/40th of an inch thick.7
Although these mattresses provide significant clinical benefits, they also have certain limitations. The EPA’s List K (Table 1 in the appendix) shows that most microbicidal agents, including those aimed at C. diff, are specifically formulated for hard surfaces, rendering them unsuitable for modern mattresses.8a Using these disinfectants can damage the mattress cover, allowing fluids and moisture to seep into the mattress core, potentially resulting in damage that undermines their ability to prevent pressure injuries.9
In addition to the fact that no EPA List K disinfectant is meant for soft, porous surfaces, Thurman points out that most mattress manufacturers recommend using a disinfectant with a pH of 5-9 to avoid damaging the surface materials. Yet the recommended dilution of bleach to kill C. diff results in a pH of 11-13.9 One major mattress manufacturer just recalled mattresses to reissue reprocessing instructions that limit the use of bleach to six times over the life of the mattress cover.10
aLists provided by the EPA are not inclusive of all products that may qualify
Aggressive Disinfection to Address Healthcare-Acquired Infections(HAIs)
HAIs are another CMS-declared “never event.”5 Virulent MDROs such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and C. diff are proliferating, and biofilms that encapsulate the pathogens are making them more difficult than ever to eradicate.11 To prevent the spread of these MDROs and the infections they cause, health care facilities have intensified their disinfection efforts. Improperly used disinfectants can erode the waterproofing of mattresses and allow for cross-contamination, putting patients at risk for a CMS “never event.”
Numerous studies link patient infections to the previous mattress occupant. Cohen et al. found that a patient is 5.83 times more likely to contract an HAI if the previous bed occupant had an infection.12
Health care facilities are also enhancing their disinfection efforts, recognizing the risks posed by asymptomatic C. diff patients, who can contaminate environments and endanger future room occupants. Witt’s research showed that mattresses used by C. diff patients can remain a source of contagion 90 days later, even after multiple rounds of cleaning and disinfection. This has led to heightened cleaning and disinfection efforts for mattresses that facilities might otherwise regard as non-critical medical devices (which require a lower level of disinfection).13,14
The Push for Quicker Room Turnover After Terminal Discharge
Facilities face pressure to reduce the time to terminally clean rooms at discharge in order to enhance patient throughput. Many operate under the misunderstanding that a one-step, wipe-and-walk approach is adequate for disinfection. According to a 2019 survey by the Association for Professionals in Infection Control and Epidemiology (APIC), 86% of infection preventionists indicated that their hospitals employ a wipe-and-walk method for mattress reprocessing between patients.15

Despite mattresses being considered non-critical medical devices, they still require intermediate- level disinfection due to potential contact with blood and bodily fluids. Intermediate disinfection as defined by the CDC is a process that removes 99.9999% of vegetative bacteria and 99.9% of mycobacteria.14 However, it is important to note that the FDA does not require the device manufacturer to validate a process for removing C.diff spores.16 According to their instructions for use, hospital bed manufacturers require a 5-6 step process to achieve this level of disinfection. Figure 2 illustrates these steps with cleaning and disinfection being distinctly different processes. Dwell time is a crucial aspect of efficacy, and rinsing mattresses with water is necessary.15,16
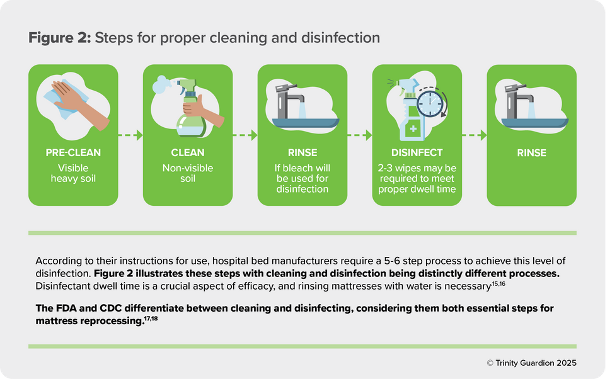
The FDA and CDC Differentiate Between Cleaning and Disinfecting Considering them both Essential Steps for Mattress Reprocessing 17, 18
The wipe-and-walk approach jeopardizes sufficient disinfection because it does not clean before disinfection, does not allow adequate dwell time and does not include a rinse and dry step. This approach also contributes to mattress breakdown, which can occur in less than two years.7 Yet, adding these steps slows turn time by approximately 20-40 minutes.
Reprocessing mattresses is complicated for Environmental Services (EVS) staff, as each manufacturer specifies different disinfectants per their MIFU. This means EVS may need to stock multiple disinfectants and change products based on the bed type. The ECRI Institute acknowledges these discrepancies and suggests informing EVS about the bed type and required disinfection level.19
Manufacturers caution against mixing disinfectant technologies without rinsing, as off-gassing may occur, creating a safety issue for staff. Residual disinfectants remaining on patient surfaces can lead to skin damage.20
This wipe-and-walk method does not guarantee proper disinfection. Numerous studies have shown that mattresses remain contaminated even after terminal cleaning.16,21
A prospective multicenter trial involving 23 acute care hospitals by Carling, Parry and von Beheren identified the thoroughness of terminal cleaning averaged 49% of surfaces.13 In a separate MRSA study, Blythe et al. reported environmental contamination in the rooms of 73% of infected patients and 69% of colonized patients.14
Hooker collected samples from 38 hospital beds using only traditional sheets during patient use to determine the level of mattress contamination after terminal cleaning. Their results revealed an insufficient reduction in FDA-required disinfection of vegetative bacteria. Additionally, they identified several pathogens in their samples known to significantly contribute to HAIs, including MRSA and VRE. This cleaning and disinfecting failure can lead to an increased transfer of MDROs and infections to subsequent patients.21

In addition, the wipe-and-walk method is not included in mattress MIFUs. CMS requires MIFU compliance as part of its Condition of Participation agreement with a hospital. The Joint Commission has also reinforced the importance of following the MIFU for mattress reprocessing in its guidelines effective 7/1/2024.22 Details of the Joint Commission guidance and other regulatory considerations are included in Table 2 in the appendix.
Not following the instructions for use voids the manufacturer’s warranty and can negate the clinical benefit being sought by the original mattress selections due to mattress failures.17,18 One manufacturer has stated: “If a patient is not removed from a damaged surface, this defect (leaking mattress) may also lead to patient exposure to excessive moisture and/or pressure, shear or friction of bony prominences, which may cause discomfort and increase the risk of skin breakdown or pressure ulcers.” 23
Whack-A-Mole: The Unintended Consequences of Doing the Right Thing
Once a mattress integrated cover has failed and fluids permeate the mattress core, the evaporative capacity is altered and the clinical benefit diminished.24
All the steps health care leaders take in the interest of better patient care have unintended consequences that undermine these very initiatives. Softer, more permeable mattresses and the increased use of harsh disinfectants to combat MDROs, along with pressure to decrease room turnover times, have caused mattress covers to wear out faster than they should. Damaged mattresses allow bodily fluids and bacteria to seep in and out of the mattress core, making decontamination impossible. This creates another significant patient safety issue while also compromising the clinical benefit of modern mattresses.7


A 2021 peer-reviewed study evaluated 727 mattresses across four midwestern hospitals. Researchers found 72% of mattresses were damaged. Of these, 47% needed the mattress cover replaced, and 25% required the entire mattress (cover and core) replaced. 7% of the mattresses that needed to be replaced were less than one year old.7
In a Medline-sponsored study, 5,121 mattresses were evaluated across 85 facilities. 59% of mattresses were red-tagged, with holes or tears, stains or fluid exudation as common issues. 25
In a 2019 survey conducted by APIC, 52% of infection preventionists reported mattresses leaking a previous patient’s bodily fluids–68% of those reporting multiple fluid leakages.15
In the last 24 months, organizations involved in direct patient care have brought the mattress conversation forward with evidence that this is a real-world issue.26-28
In November 2022, the Washington State Nurses Association (WSNA) filed a complaint with the Washington State Department of Health, citing mattresses as a possible biohazard risk for patients after nurses in a labor and delivery unit discovered blood and bodily fluids seeping from the mattress covers in patients’ beds.26 According to the news report, the hospital knew about the issue and tried to patch the damaged mattresses but ran out of patching material. Applying a patch to damaged mattresses is not an FDA-cleared solution and does not protect patients from the potentially contaminated mattress core.26
Most recently, the Veterans Health Administration (VHA) issued patient safety alert SR-46567 in April of 2024, declaring mattress fluid ingress and egress a patient safety issue. The Veterans Affairs (VA) patient safety alert specifically calls out that “mattresses can lead to hospital-associated infections, patient tissue degradation and/or patient death.” 29
Patients are Taking Matters into Their Own Hands
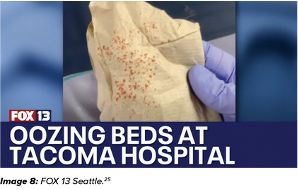
It’s not just health care professionals; patients are aware of the problem and taking action. In 2024, two patients used their cell phones to record the state of their hospital mattresses and shared the videos with local news stations. As a result, these broadcasts damaged the facility’s reputation. (References include links.)30,31
Call for Action
Health care leaders must urgently prioritize patient safety by addressing mattress reprocessing with the same dedication given to hand hygiene, ensuring strict compliance with established standards. A critical first step toward progress is openly discussing the trade-offs between pressure injuries, HAI disinfection, room turnover time, patient throughput and mattress reprocessing or breakdowns.
Leaders must also hold the industry accountable for innovation that minimizes these trade-offs and enhances patient safety.
Addressing this clear unmet need is essential to safeguard patients, health care providers and staff from damaged mattresses.
Appendix
EPA’s Registered Antimicrobial Products Effective Against C. diff Spores – https://www.epa.gov/pesticide-registration/epas-registered-antimicrobial-products-effective-against-clostridioides
The link above illustrates that disinfectants are not designed for soft, porous surfaces. However, depending on the manufacture’s reprocessing IFU, disinfectants can be used. A facility may need multiple disinfectants in its supply closet because manufacturers validate specific disinfectants for different beds. Recognizing the impact of disinfectants on these soft support surfaces, manufacturers have begun to adjust MIFUs to limit the number of times a disinfectant can be used over the life of the integrated mattress cover.
Table 2: Regulatory Considerations
NEW JOINT COMMISSION STANDARD IC.06.01.01 EP322
Undamaged hospital bed mattress covers ore cleaned and disinfected according to manufacturer’s instructions for use.
Key Elements:
Damaged, worn or visibliy stained hospital bed mattress or mattress covers are removed from service and cleaned, disinfected, refurbished or discarded in accordance with the manufacturer instructions and hospital procedures.
MIFU components include:
- Multi-step cleaning and disinfection process
- Verification that integrated mattress cover has not exceeded expected life
- External mattress inspection after each patient discharge
- Periodic internal mattress inspections
JOINT COMMISSION STANDARD IC.06.01.01 EP3 / CMS STANDARD 2.D4
OSHA VIOLATIONS32
Cleaners and disinfectants, including disposable wipes, are used in accordance with manufacturers’ instructions (for example, dilution, storage, shelf-life, contact time).
- Use of a wipe and walk disiinfection process is not iin accordance wiith the use of a hard surface diisin·fectant EPA approved claims
- 1910.10030(b) Engineering controls that remove blood borne pathogens from the workplace
- 1910.1310(d)(1) Precautions to prevent exposure to potentially intectiion materials
- 1920.1030(d)(4)(ii)9A) Contaminated work surfaces shall be decontamiinated with appropriate disinfectant
Relevant Joint Commission/CMS Crosswalk Standards
JOINT COMMISSION STANDARD 2 CMS CROSSWALK22
IC.06.01.01 EP3
The hospital implements activities for the surveillance, prevention, and control of health care-associated infections and other infectious diseases, including maintaining a clean and sanitary environment to avoid sources and1ransmission of infection, and addresses any infection control issues identified by public health authorities
§482.42 Condition of Participation
Infection prevention and control and antibiotic stewardship
- Hospital Average Length of Stay by State. Definitive Healthcare. Accessed November 15 2024, https://www.definitivehc.com/resources/glossary/length-of-stay#:~:text=Length%20of%20stay%20(LOS)%20is, (heart%20attacks)%20and%20diabetes.
- Marks B, de Haas E, Abboud T, Lam I, Datta I. Uncovering the rates of damaged patient bed and stretcher mattresses in Canadian acute care hospitals. Canadian Journal of Infection Control. 2018;33(3):171-175.
- Furness CD, Srigley JA, Gardam M. How much do beds and mattresses sleep around? Automated measurement of bed frame and mattress movement in an acute care hospital. Canadian Journal of Infection Control. 2017;32(4):222-224.
- Considerations for Reducing Risk: Surfaces in Healthcare Facilities Centers for Disease Control and Prevention. Accessed November 15, 2024, https://www.cdc.gov/healthcare-associated-infections/hcp/infection-control/index.html
- ELIMINATING SERIOUS, PREVENTABLE, AND COSTLY MEDICAL ERRORS – NEVER EVENTS. Accessed February 11, 2025. https://www.cms.gov/newsroom/fact-sheets/eliminating-serious-preventable-and-costly-medical-errors-never-events
- Miller MW, Emeny RT, Freed GL. Reduction of Hospital-acquired Pressure Injuries Using a Multidisciplinary Team Approach: A Descriptive Study. Wounds. Apr 2019;31(4):108-113.
- Hooker EA. Hospital mattress failures—A hidden patient danger. Infect Control Hosp Epidemiol. 2023;44(3):501-503. doi:10.1017/ice.2021.486
- EPA’s Registered Antimicrobial Products Effective Against Clostridioides difficile (C. diff) Spores [List K] United States Environmental Protection Agency. Updated June 3, 2024. Accessed December 1, 2024. https://www.epa.gov/pesticide- registration/epas-registered-antimicrobial-products-effective-against-clostridioides
- Thurman K, Todd J, Ambrose-Jones S. Impact of Cleaning and Disinfecting on Full-Body Support Surfaces. Adv Skin Wound Care. Jan 29 2025;doi:10.1097/asw.0000000000000268
- Class 2 Device Recall Pro attress. U.S. Food and Drug Administration. Accessed December 9, 2024. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=206541
- Prinzi A, Rohde R. The Role of Bacterial Biofilms in Antimicrobial Resistance. The American Society for Microbiolog. Accessed November 18, 2024. https://asm.org/articles/2023/march/the-role-of-bacterial-biofilms-in-antimicrobial-re
- Cohen B, Liu J, Cohen AR, Larson E. Association Between Healthcare-Associated Infection and Exposure to Hospital Roommates and Previous Bed Occupants with the Same Organism. Infect Control Hosp Epidemiol. May 2018;39(5):541-546. doi:10.1017/ice.2018.22
- Witt LS, Howard-Anderson J, Prakash-Asrani R, Overton E, Jacob JT. The role of the hospital bed in hospital-onset Clostridioides difficile: A retrospective study with mediation analysis. Infect Control Hosp Epidemiol. May 2024;45(5):599-603. doi:10.1017/ice.2023.254
- Recommendations for Disinfection and Sterilization in Healthcare Facilities. Centers for Disease Control and Prevention. Updated June 2024. Accessed November 15, 2024, https://www.cdc.gov/infection-control/hcp/disinfection-and- sterilization/index.html
- Hooker EA. Disinfecting hospital beds and mattresses: A time for change. American Journal of Infection Control. 2021;49(10):1341. doi:10.1016/j.ajic.2021.07.020
- Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling Guidance for Industry and Food and Drug Administration Staff. U.S Food & Drug Administration. Updated June 9, 2017. https://www.fda.gov/media/80265/download
- IsoFlex LAL® Support Surface Instructions For Use. Stryker Medical. https://techweb.stryker.com/Support_Surfaces/2860/IsoFlex/2860-109-001C.2.pdf
- P280 Mattress Instructions for Use. Hill-Rom Services, Inc. Updated October 2020. Accessed December 1, 2024. https://www.hillrom.co.uk/content/dam/hillrom-aem/emea/en/marketing/products/p280- mattress/documents/187692(10)_EN.pdf
- Reducing the Risks of Fluid Ingress and Microbiological Contamination in Bed and Stretcher Support Surfaces. ECRI Institute. Updated May 10th, 2017. Accessed February 18th, 2025.
- Virex® II 256 SDS # MS0801116. Diversey, Inc. Updated August 08, 2024. Accessed February 18, 2025.https://sds.diversey.com/DirectDocumentDownloader/Document?prd=MS0801116~~PDF~~MTR~~ANEP~~EN~~
- Hooker EA, Allen S, Gray L, Kaufman C. A randomized trial to evaluate a launderable bed protection system for hospital beds. Antimicrobial Resistance and Infection Control. 2012/07/26 2012;1(1):27. doi:10.1186/2047-2994-1-27
- Infection Prevention and Control Program Assessment Tool. The Joint Commission. Accessed February 18, 2025.
- Baxter—Centrella and pro+ Mattress Replacement System Hospital Bed Mattresses: Top Cover May Delaminate. ECRI Institute. Accessed February 18, 2025.
- Class 2 Device Recall Centrella Max and pro. U.S. Food and Drug Administration. Accessed December 9, 2024. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRES/res.cfm?id=207675
- Koshy T, Manista G, Nicholson L, Ikpeze T, Todd J, Black J. The State of Support Surface Integrity in Acute Healthcare Facilities. presented at: National Pressure Injury Advisory Panel (NPIAP); 2023; San Diego, California.
- Wong N. Mattresses with bodily fluid found in labor and delivery units at St. Joseph’s Medical Center. FOX13 Seattle.
- 2019 Top 10 Health Technology Hazards Executive brief. ECRI Institute. February 18, 2025. https://www.ecri.org/resources/whitepapers_and_reports/haz_19.pdf
- Status Implementation of Resolutions and Report Recommendations AMA House of Delegates Meeting – June 2023. American Medical Association. Accessed December 9, 2024. https://www.ama-assn.org/system/files/a23-status.pdf
- Policy Proceedings of the 2023 annual meeting of the AMA organized medical staff section. American Medical Association. December 9, 2024. https://www.ama-assn.org/system/files/a23-omss-policy-proceedings.pdf
- Krafcik M. Kalamazoo woman unknowingly sits in urine-soaked hospital bed. Accessed February 18, 2025. https://wwmt.com/news/local/hospital-bronson-urine-dirty-bed-kalamazoo-michigan-healthcare-patient-sheets-pee- investigation-dirty-code
- Searcy L. Woman calls for change after taking a closer look at hospital beds. Lex18 Lexington Accessed February 18, 2025. https://www.lex18.com/news/lex-18-investigates/woman-calls-for-change-after-taking-a-closer-look-at-lexington-hospital-beds
